

Young puppies infected with canine distemper virus (CDV) before the eruption of permanent dentition may show severe changes in the crowns or roots. Enamel hypoplasia may be an incidental finding in an older dog and it is relatively pathognomonic of previous infection with CDV. CDV belongs to the family Paramyxoviridae. A seven-month-old spayed female stray dog was presented for long-term treatment of four upper incisors and the upper right canine tooth. The teeth showed relatively pathognomonic morphologies of the crowns for a previous infection with CDV. Most likely, the patient went through a subclinical episode of distemper at the age of three months. The owners expressed concern about the circular defects on the crowns of the canine tooth and incisors. The therapeutic approach was based on the principle of “no suffering in silence”, long-term success, minimal dental intervention and economic aspects. As the dental pulp of the canine tooth was in a proper condition to be collected, it was placed in neutral buffered formalin (NBF) immediately after surgical extraction. Multiple faintly eosinophilic intra-
nuclear viral inclusions were identified mostly in the fibroblasts of the pulp core and occasionally in the odontoblasts and cells of the cell-rich zone. Random intranuclear viral inclusions with a size of 1-5µm could even be found in the endothelial cells of the blood vessels of the pulp core.
Keywords: Canine dental pulp, enamel hypoplasia, canine distemper virus, inclusion bodies, veterinary dentistry
Aim of the case report: Our case reports a histological aspect of eosinophilic inclusion bodies in the canine dental pulp’s cells. The inclusion bodies were eosinophilic, both nuclear and intra-
cytoplasmic, resembling very close to the CDV inclusion bodies found in other parts of an infected body.
CDV was first described by Henri Carre in France in 1905 (6).
CDV belongs to the family Paramyxoviridae, subfamily Paramyxoviridae, genus Morbillivirus. It is closely related to human measles virus (HMV) and rinderpest virus (RPV), as they are all morbilliviruses (17). CDV infects terrestrial and aquatic mammals worldwide. Domestic dogs infected with CDV show signs of upper respiratory tract disease or gastroenteritis. Clinical signs vary in different patches, although CDV is serologically homogeneous with little genetic variation. Infected dogs may undergo a subclinical infection or show a rapidly progressive infection with subsequent exitus.
These heterogeneous manifestations are related to the age and immune status of the host and association with other viruses or bacteria. After entry into the host, CDV initially infects the monocytes of the upper respiratory tract and spreads via the lymphatics and blood to the reticuloendothelial system. CDV also spreads to epithelial tissue and the central nervous system (15). Young puppies infected with CDV before the eruption of permanent dentition may show severe changes in the crowns or roots. Enamel hypoplasia may be an incidental finding in an older dog and is relatively pathognomonic of previous infection with CDV (4). These dental lesions were studied only in association with ameloblasts and amelogenesis due to the epitheliotropic nature of the virus. CDV thus directly infects and destroys the ameloblasts (16). The enamel defects caused by CDV range from small localized depressions to large areas without enamel, often with discrete borders (2). Some authors (16) report circumferential areas without enamel rather than lines or depressions. All studies to date have focused on the enamel organ and the lesions that developed as a result of CDV acquired prior to the final mineralization of the enamel organ. No study has examined the pulp tissue in teeth with lesions resulting from infection with CDV. Our case report opens new perspectives upon correlations between viral infections in young childhood and the pulp tissues. More studies are warranted.
Materials and methods
A seven-month-old female spayed mixed-breed dog was presented for treatment of the right maxillary canine tooth (104) and four of the maxillary incisors’ teeth (103, 102, 101, 201). Awake examination of the affected teeth revealed two circular grooves in the incisal third of the crowns. Due to the lack of a continuous enamel structure in the crown and the fact that incisors are teeth used for prehension and incision of the food, the 201 (left upper incisor) was fractured at the level of the first groove. The owners assumed that the fractures at the levels of the grooves would also occur in the other teeth in the near future. Therefore, they contacted the veterinary dental services. The long-term accepted method was closed or surgical extractions (depending on the local conditions) of the affected teeth. Pre-operative complete blood count and biochemistry panels revealed no contraindications for general anaesthesia. Temperature, heart rate and respiratory rate were within normal limits.
Results | Clinical and radiological findings
A thorough examination of the oral cavity, under general anesthesia and with the help of dental X-rays revealed no other evidence of root developmental defect of endodontic/periodontal disease on the other remaining teeth. The offended teeth had two circular grooves in the incisal third of the crowns. In 104, the first circular groove was located 3 mm from the tip of the crown. In the incisors, the first groove was located 2 mm from the tip of the crown. The dimensions of the grooves were one millimeter wide and one millimeter deep (Figure 1).
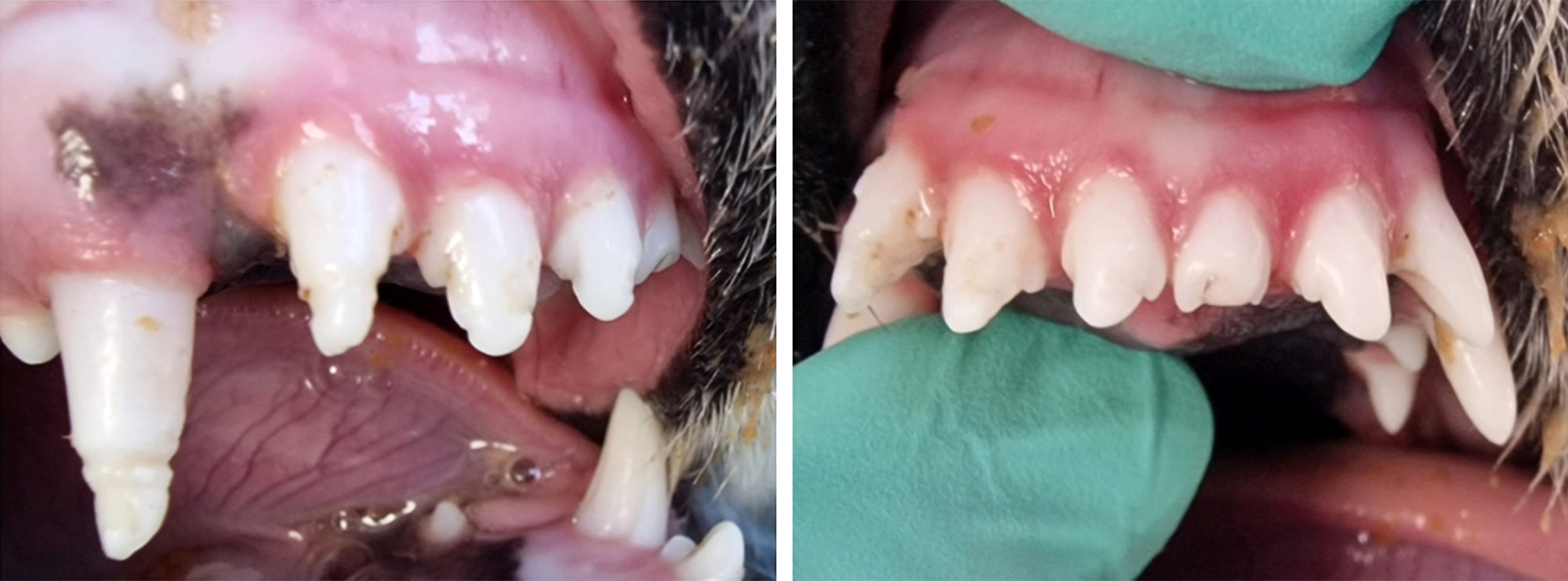
Figure 1 | 104, 103, 102, 101 with the two grooves located in the incisal third of the crowns. 201 has an uncomplicated coronal fracture. Intraoral dental radiographs suggested the age of seven-months with large pulp chambers and thin surrounding dental hard tissues. Postoperative dental radiographs confirmed complete exodontia. A large root canal and a large apex of the root was observed on the dental X-rays. The devoid enamel was the only abnormality that could be identified. The macroscopic aspect of the crown suggested a potential previous infection with CDV. There was no intrinsic or extrinsic discoloration. The crown was snowy white, without any pink or other shade colors. None of them were intra-vitam pink teeth. The measurements of the defects mentioned above were confirmed with the digital application for dental X-rays. (Figure 2)
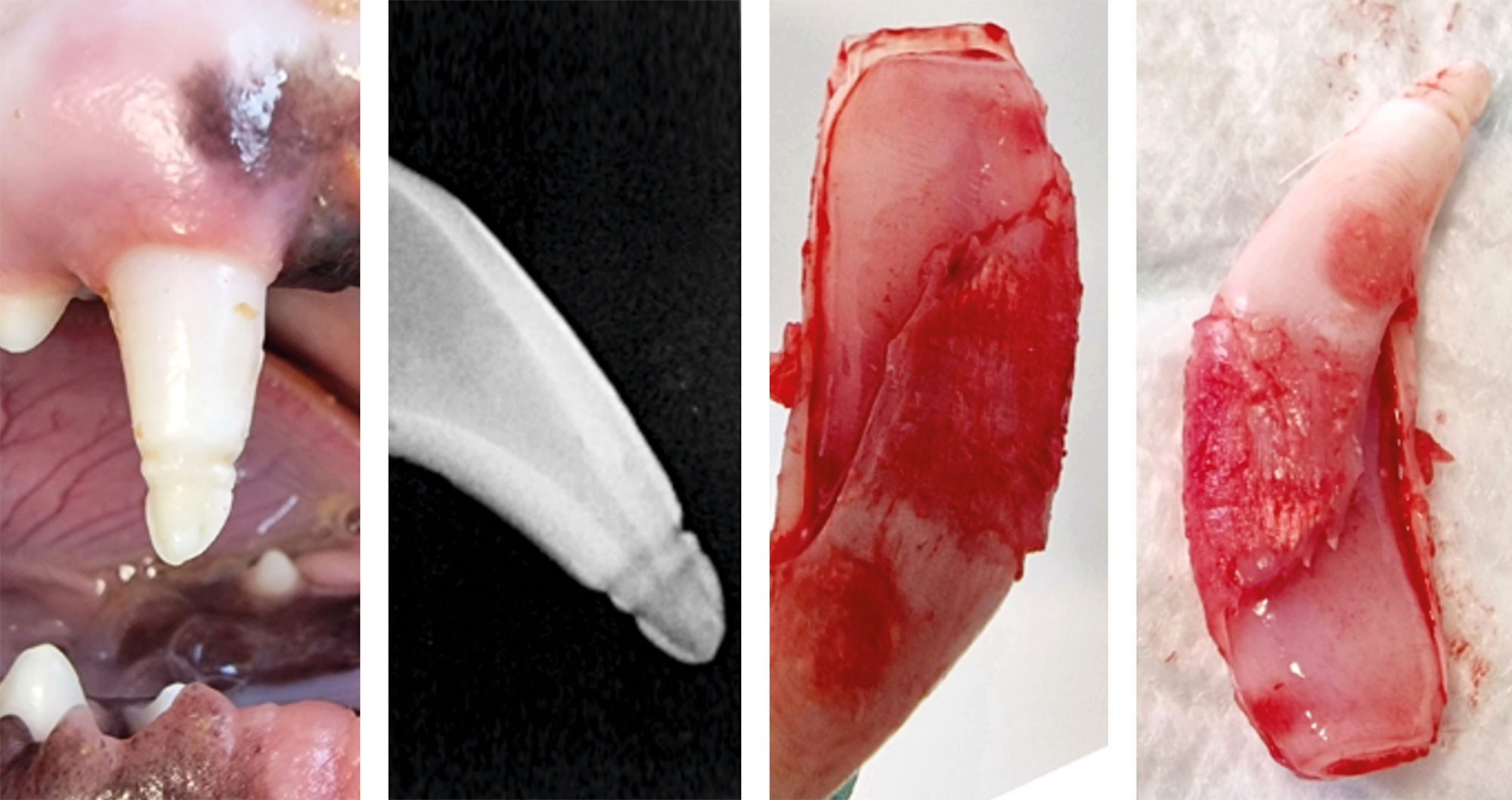
Figure 2 | Clinical and radiological aspect of the canine tooth’ crown, and clinical post extraction aspect of the dental pulp. Note the large pulp chamber and the apex closure. The dental pulp showed no signs of hyperemia, necrosis or any other dental pulp pathologies. Immunization of the patient started at the approximate age of two months with a vaccine consisted of live, attenuated CDV and combined with attenuated canine parvovirus vaccine. After two weeks the vaccine consisted of live, attenuated CDV, and attenuated adenovirus type 2, parvovirus and parainfluenza virus. After another two weeks the patient received live, attenuated CDV, adenovirus type 2, parvovirus, parainfluenza virus and Leptospira and it was repeated at fourteen days. The neurologic examination revealed no signs of injury or disease of the brain, cerebrum, diencephalon, vestibular, cranial nerves, dysfunction in walking or in any other reflexes.
Surgical approach
The owners opted for dental extractions as a therapeutic approach. They wanted a relatively safe, long-term solution to ensure that their dog would not suffer of pain in the future because of the affected teeth. In the meantime, they did not want to undergo multiple general anaesthesias. The dog was induced with propofol (4mg/kg i.v.) and maintained with isoflurane (1.3%) after the endotracheal tube was placed. The right infraorbital nerve block was performed via an intraoral access. Lidocaine 2% 0.5 ml/site (13) was used for local anaesthesia. As the dog was young, and the maxillary bones were easily expandable, closed extractions were able to be performed with minimal trauma of the surrounding tissues. The sutures were placed in an interrupted pattern with three throws to ensure knot security with synthetic, absorbable 4.0 USP monofilament (12). Postoperative treatment consisted only of meloxicam oral suspension, a non-steroidal anti-inflammatory drug, administrated for pain management, 0,1mg/kg bodyweight. Gingival healing was achieved at two weeks. Sutures were resorbed completely at six weeks from the surgical intervention. No lip entrapment occurred. No other post-operative surgical complications are to be mentioned. There was no food restriction for the patient.
Histological findings
In the next 10 minutes following extraction, the dental pulp of the canine tooth was fixed in NBF.
It was then, histologically processed by paraffin embedding technique. Later, the sections were stained Goldner’s trichrome (GT) method and examined under an optical microscope.
The histological appearance of the dental pulp under low-power examination (i.e., objective 4x) in a longitudinal section distinguished the four zones found in the dental pulp (11): odontoblasts, a cell-free zone of Weil, the cell-rich zone and centrally a highly vascularized pulp core. One detail stands out at this point: the cell-free zone is present, but greatly reduced (Figure 3).
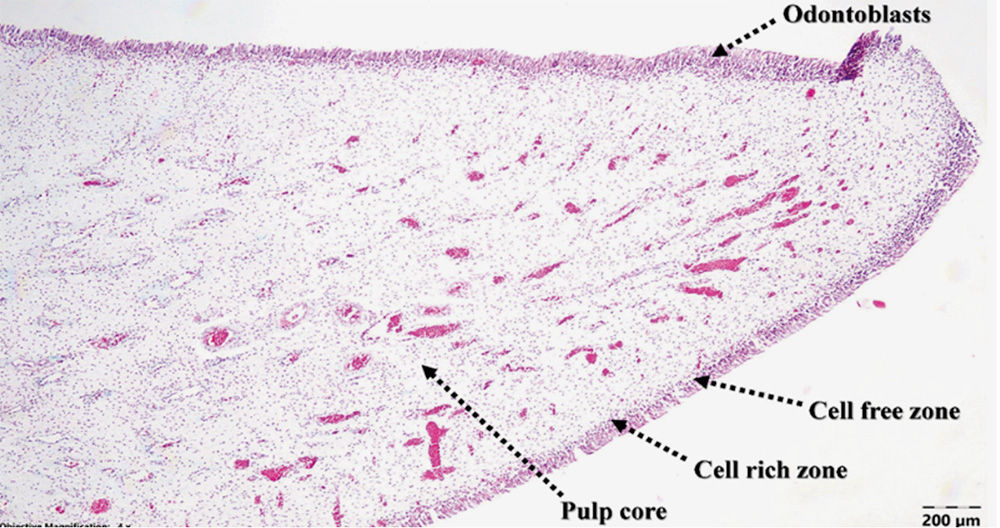
Figure 3 | Canine dental pulp structure under 4x magnification, TG staining. At higher power magnification (i.e., objective 40x), multiple faintly eosinophilic intranuclear viral inclusions were identified mostly in the fibroblasts of the pulp core and occasionally in the odontoblasts and cells of the cell-rich zone (Figure 4).
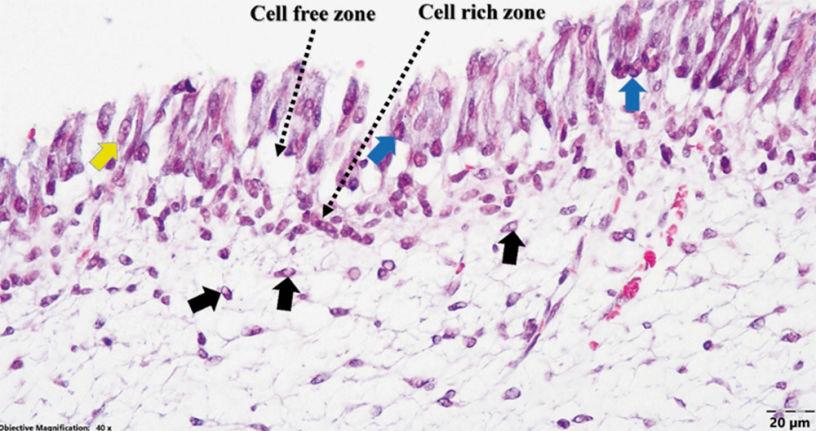
Figure 4 | Dental pulp (dog presumed previously infected with CDV; Goldner’s trichrome stain): numerous faintly acidophilic intranuclear viral inclusions in fibroblasts of the pulp core (black arrows), and occasional intranuclear (blue arrows) and intracytoplasmic (yellow arrow) inclusions in odontoblasts. Random intra- nuclear viral inclusions with a size of 1-5µm could even be found in the endothelial cells of the blood vessels of the pulp core. (Figure 5)
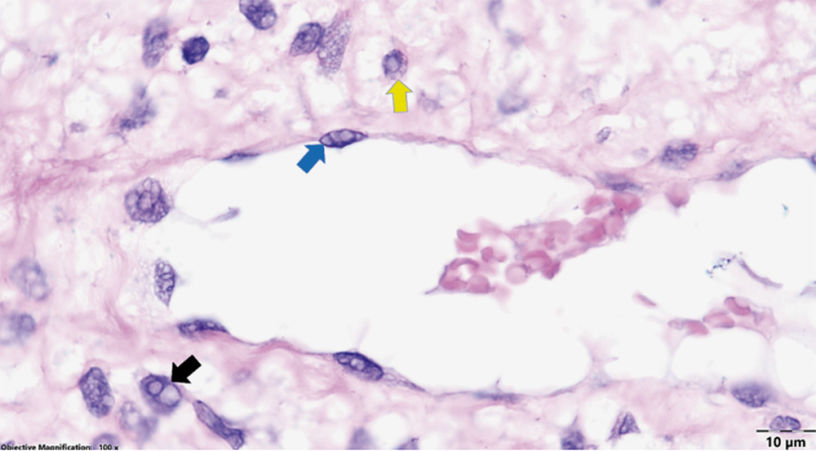
Figure 5 | Dental pulp of a dog presumed to be infected with CDV (hematoxylin-eosin staining): singular or several intranuclear (black arrows) and/or occasional intracytoplasmic (yellow arrow) viral inclusions in fibroblasts of the pulp core, and seldom intranuclear inclusions in the endothelial cells (blue arrows).Immunohisto-chemical examination for CDV was performed. The results were negative for CDV infection. According to the laboratory this might also indicate that the expression is very low, and below the immunohistochemical detection threshold. RT-PCR had the same results and mentions as immunohistochemistry.
Discussion
The medical team outlined the five therapeutic approaches for this case. The first would be to continue monitoring for signs of pain or complicated crown fractures. It was rejected by the owners.
The second would be to fill the grooves with light-curing composite. Given the fact that the incisor teeth and the canine tooth are teeth involved in prehension, incision and tearing, a small layer of composite would not offer a stable, long-term therapeutical solution. Most surely, it would be chopped or fallen out in a variable period of time involving another general anaesthesia for our patient. Beside the medical aspect of several general anaesthesias to be performed, the economic aspect is not to be neglected. The third restorative solution in these pathologies are prosthetic crowns. Prosthodontic crowns would involve preparing the teeth, involving or not an endodontic treatment, a session for impression and a session for applying the crowns. The costs would be higher, and would imply two general anaesthesias and additionally radiological surveillance annually. Dental X-rays in veterinary dentistry are obtained in general anaesthesia. Again, a dental treatment rejected by the owners. The fourth therapeutic approach, according to Boy et al., would be application of a dental sealant or bonding. These applications need to be renewed every six months. Owner refused this approach. Smoothing of the teeth was rejected by the medical team as it would have compromised the resistance of the tip of the affected teeth and would have sensibilized the teeth. The long-term accepted method was closed or surgical extractions (depending on the local conditions) of the affected teeth.
Mineralization of the entire coronal part of the permanent dentition occurs at about three to four months of age in dogs (1). Since the root of the canine tooth had the apex opened, we can estimate the age of our patient to be 180 days (10,18). From the authors’ personal experience, there is a short delay between the maturation of the teeth between the right and left quadrants of the maxilla and mandible. Distemper is more frequent in dogs between 3 and 6 months of age. We can presume that the subclinical infection occurred before the end of amelogenesis, around three to four months of age. Brachycephalic dogs have a lower prevalence of disease, mortality and sequelae than dolichocephalic breeds (16). Our patient was a mixed medium-sized dolichocephalic dog, consistent with previous findings. Studies on hypersensitivity caused by enamel defects (9) have shown that these affected teeth can be painful and develop suffering over time. Animal patients are known to endure dental pain in silence, and dogs hide their pain for long periods of time. Enamel defects caused by CDV were described previously as generalized lesions involving enamel hypoplasia of the maxillary and mandibular canine teeth, incisors, and premolars; and dentin hypoplasia of the maxillary incisors, maxillary premolars, and mandibular premolars (2). All studies to date have focused on the enamel organ (5) and the lesions that developed as a result of CDV acquired prior to the final mineralization of the enamel organ. No study has examined the pulp tissue in teeth with lesions resulting from infection with CDV. The negative results of immunohisto-chemical examination and RT-PCR may be due to the fact that low quantities of the virus are present in the specimens. Immunostaining may be less rewarding because of antibody coating, in chronic cases. Additionally, it is accepted that the virus persists for at least 60 days after infection in the skin, uveal tissue, footpad and central nervous system. (16) As no one has studied before the potential presence of CDV in the dental pulp, no one could tell whether the IHC or RT-PCR is relevant from the dental pulp or not at this stage of the case. A negative result doesn’t clarify whether the inclusion bodies belong to CDV. They only suggest the negative estate at this time. The cell-free zone is a term that involves certain discussions regarding the canine dental pulp. No previous studies have clarified whether dogs have or not this histological pulp layer. The authors did not interpret as a pathological aspect, but it needs to be mentioned a narrow cell-free zone that could be observed on the histological samples, under the odontoblasts. The cell-free zone is stated as absent in equine teeth (14) and rats’ teeth (7). The cell-free zone is present in human teeth and non-human primate teeth (11). Further studies regarding this histological aspect are warranted.
The neurotropism of CDV and the induced cell necrosis of the epithelial cells are well known. Less frequently, CDV affects cells of the connective tissue. The enamel organ develops from the ectoderm of the first dental arch. The dental pulp, on the other hand, is formed from the ectomesenchyme of the neural crest (11). Considering the two different origins of the affected tissues and their effects on tooth development, which correlates with the new findings, further studies are warranted.
Conclusions
The synchronization of defects in this group of teeth associated with the histological aspects suggest a previous infection with CDV. The repetitiveness of the enamel lesions and the fact that they were made at approximately the same levels compared to the tip of the crown exclude the possibility of a traumatic injury. Our case report opens new perspectives regarding the viral inclusions present in the canine dental pulp’s tissues.
References
1. Arnall L, Some aspects of dental development in the dog- I Calcification of the crown and root of the deciduous dentition. (1961) Journal of Small Animal Practice. 1, 169-173.
2. Bittegeko SB, Arnbjerg J, Nkya R, Tevik A. Multiple dental developmental abnormalities following canine distemper infection. J Am Anim Hosp Assoc (1995) 31:42–5. doi:10.5326/15473317-31-1-42
3. Boy S, Crossley D, Steenkamp G. Developmental Structural Tooth Defects in Dogs - Experience from Veterinary Dental Referral Practice and Review of the Literature. Front Vet Sci. 2016 Feb 8;3:9. doi: 10.3389/fvets.2016.00009. PMID: 26904551; PMCID: PMC4744861.
4. Carvalho OV, Botelho CV, Ferreira CG, Scherer PO, Soares-Martins JA, Almeida MR, Silva Júnior A. Immunopathogenic & neurological mechanisms of canine distemper virus. Adv Virol. 2012;2012:163860. doi: 10.1155/2012/163860. Epub 2012 Nov 4. PMID: 23193403; PMCID: PMC3501799.
5.Dubielzig RR, Higgins RJ, Krakowka S. Lesions of the enamel organ of developing dog teeth following experimental inoculation of gnotobiotic puppies with canine distemper virus. (1981). Vet Pathol. 18: 684-689.
6. Gamiz C, Martella V, Ulloa R et al. Identification of a new genotype of canine distemper virus circulating in America. Vet Res Commun. (2011); 35: 381-390.
7. Gotjamanos T. Cellular organization in the subodontoblastic zone of the dental pulp-(: A study of cell-free & cell-rick layers in pulps of adult rat & deciduous. Arch Oral Biol. 1969 Sep;14(9):1007-1010.
8. Hillson S, Bond S. Relationship of enamel hypoplasia to the pattern of tooth crown growth: a discussion. Am J Phys Anthropol (1997) 104:89–103. 10.1002/(SICI)1096-8644(199709)104:1<89::AID-AJPA6>3.0.CO;2-8
9. Linner T, Khazaei Y, Bücher K, Pfisterer J, Hickel R, Kühnisch J. Hypersensitivity in teeth affected by molar-incisor hypomineralization (MIH). (2021) Sci Rep. Sep 9;11(1):17922. doi: 10.1038/s41598-021-95875-x. PMID: 34504122; PMCID: PMC8429747
10. Morgan JP, Miyabayashi T. Dental radiology: ageing changes in permanent teeth of beagle dogs. Journal of Small Animal Practice. (1991). 32, 11-18.
11. Nanci A, Ten Cate’s Oral Histology (2018). Chapter 8. Elsevier
12. Reiter AM. Equipment for oral surgery in small animals. Veterinary Clinics of North America Small Animal Practice. (2013). 43, 587-608.
13. Reiter AM, Gracis M. BSAVA Canine & Feline Dentistry & Oral Surgery. (2018); Chapter 6, BSAVA, UK.
14. Roßgardt J, Heilen LB, Büttner K, Dern-Wieloch J, Vogelsberg J, Staszyk C. The Equine Dental Pulp: Analysis of the Stratigraphic Arrangement of the Equine Dental Pulp in Incisors and Cheek Teeth. Veterinary Sciences. 2022; 9(11):602
15. Shina DL, Chludzinskib E, Wuc NH, Penga JY, Ciurkiewiczb M, Sawatskyd B, Pfaller CK, Baechleina C,von Messling V, Haasa L, Beinekeb A, Herrler G. Overcoming the Barrier of the Respiratory Epithelium during Canine Distemper Virus Infection. (2022) mBio. American Society for Microbiology. 13: 1-17. https://doi.org/10.1128/mbio.03043-21
16. Sykes J. Green’s Infectious diseases of the dog and cat. (2023); Chapter 22, Elsevier.
17. Uhl EW, Kelderhouse C, Buikstra J, Blick JP, Bolon B, Hogan RJ. New world origin of canine distemper: Interdisciplinary insights. Int J Paleopathol. (2019) Mar; 24:266-278. doi: 10.1016/j.ijpp.2018.12.007
18. Wilson G. Implications of the time of apical closure in relation to tooth fracture in dogs. Australian Veterinary Practitioner. (1996) 26, 65-71.
